Biology of the Nurse Cell - Parasite Complex
All information contained in this web site relates primarily to Trichinella spiralis, unless otherwise stated.
For a light-hearted look at the process of Nurse cell formation see: Trichinella spiralis: Growing Up Parasitic, the Early Years
The Nurse cell is a unique consequence of the host cell’s association with the infectious L1 larva of Trichinella spiralis and other Nurse cell-forming species of Trichinella (T. britovi, T. nelsoni, and T.nativa). It presumably functions to nourish it as well as protect it from host immune responses. The mature Nurse cell is morphologically distinct from any other mammalian cell type; no other pathological condition induces such a radically different, and yet functional cell. The Nurse cell-parasite complex can survive in the human host for up to 30 years, and in most other species of mammal for the life span of the animal. For this to occur, the worm must immunosuppress the host, yet nothing is known regarding the mechanism(s) employed by the parasite through which it keeps the host from killing it. Repeated exposure to infective L1 larvae via the oral route somehow breaks down worm-based immunosuppression and larvae from prior infections are killed. With enough repeated infections, the host is rendered sterile with regards to Trichinella.
Intravital microscopy has revealed the relationship of the Nurse cell to the surrounding tissues. The following video is an in vivo, living Nurse cell-parasite complex . The Nurse cell begins to form the moment the newborn larva penetrates the striated skeletal muscle cell.
.
Schematic of a Larva Invading a Muscle Cell
Step 1
Step 2 (Click here for photo)
Step 3 (click here for photo)
Step 4
Step 5 (Click here for photo)
Summary of Nurse Cell Formation
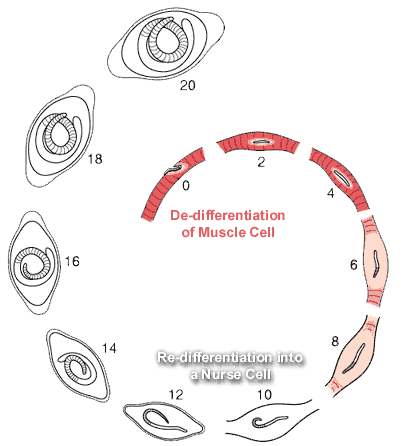
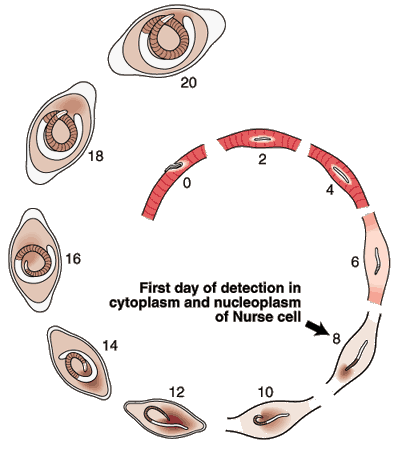
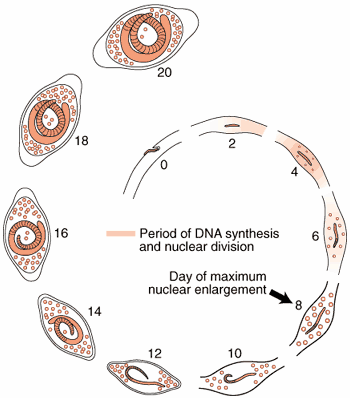
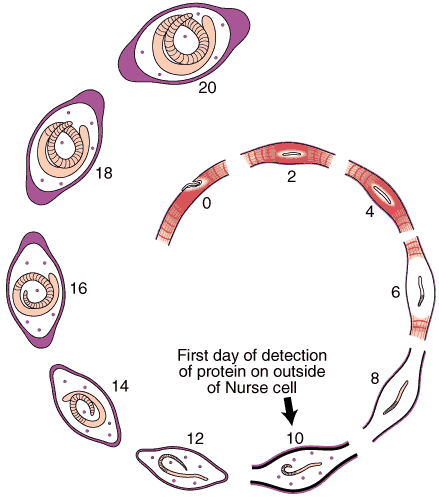
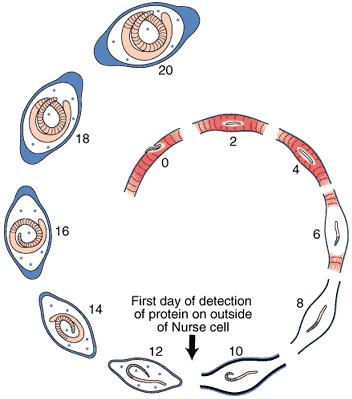
Nurse Cell Formation: De-differentiation and Re-differentiation
There are at least two distinct phases to the overall process:
1. De-differentiation of the muscle cell
2. Re-differentiation of the muscle cell into the Nurse cell
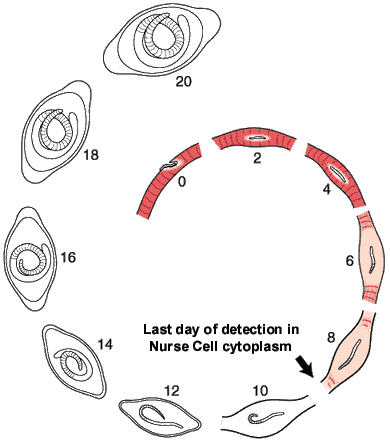
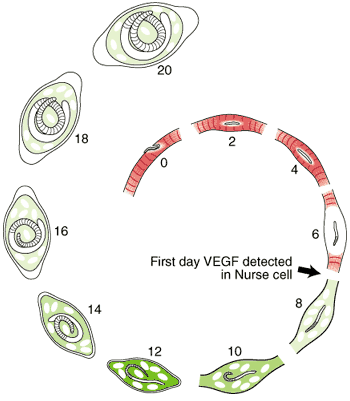
The morphological events resulting in the formation of the Nurse cell-parasite complex occur over a 14-16 day period of time after the worm has entered the muscle cell. The diagram and the graph below summarize the overall change.
1. De-differentiation of the muscle
The rate of increase in the worm’s volume is a crude measure of worm growth (see Growth Curve below), and differentiation of its tissues. For example, the stichosome, is correlated with the growth and differentiation of the Nurse cell. Only the overall growth rate of the worm has so far been documented. The larva grows during the first day after entering the muscle cell, doubling its volume. The parasite remains the same size over the next two days, resuming growth on Day 4. During this pause, the host cell becomes disorganized with respect to its contractile elements.
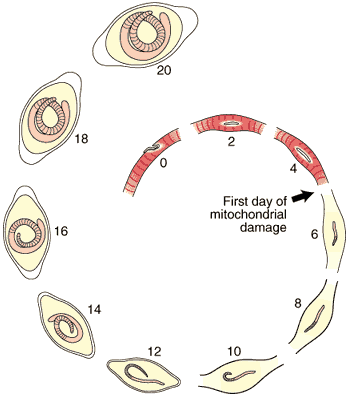
The first five days of the process of Nurse cell formation involves a loss of normal striated skeletal muscle. Actin and myosin filaments begin to disappear and the sarcolemmal membrane becomes separated from the contractile elements. Muscle cell mitochondria become vacuolated and ATP synthesis is uncoupled from aerobic metabolic pathways beginning on Day 3, and remain uncoupled thereafter for the life of the Nurse cell-parasite complex.
Up to Day 19, the parasite continues to increase its volume by 39% per day. The parasite is infective for another host on day 14-16 post-im infection.
2. Re-differentiation of infected portion of the muscle
Most of the significant changes in the infected host muscle cell are complete by Day 8 post im infection. Enlargement of host cell nuclei are at their maximum on that day (below), and it is at this point in the infection that the stichocytes begin to produce and secrete tyvelose-decorated proteins into the milieu of the infected host cell.
Normal striated skeletal muscle. Note contractile elements abut the sarcolemmal membrane. TEM
Muscle cell several days after injection of newborn larvae. Note enlarged muscle cell nucleus. TEM
Muscle cell several days after injection of newborn larvae. Note vaculolated host cell mitochondria. TEM
Muscle cell several days after injection of newborn larvae. Note disruption of cytoplasm near sarcolemmal membrane. TEM
Muscle cell several days after injection of newborn larvae. Note contractile elements in state of disarray. TEM
Muscle cell 8 days after injection of newborn larvae. Note disorganized A-Z complexes. TEM
Mature nurse cell cytoplasm 20 days after injection of newborn larvae. Note lack of contractile elements and presence of whorls of smooth membrane. TEM
Summary of nurse cell nuclear enlargement.
Enlarged muscle nucleus several days after injection of newborn larvae. H&E
Enlarged muscle cell nuclei 8 days after injection of newborn larvae. Note double nucleolus. H&E
Enlarged nurse cell nuclei 12 days after injection of newborn larvae. H&E
Enlarged nurse cell nuclei 12 days after injection of newborn larvae. H&E
Mature nurse cell nuclei 20 days after injection of newborn larvae. Note reticular appearance of nucleoli. N=4 TEM
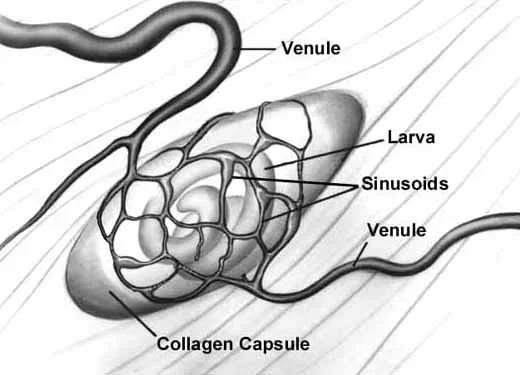
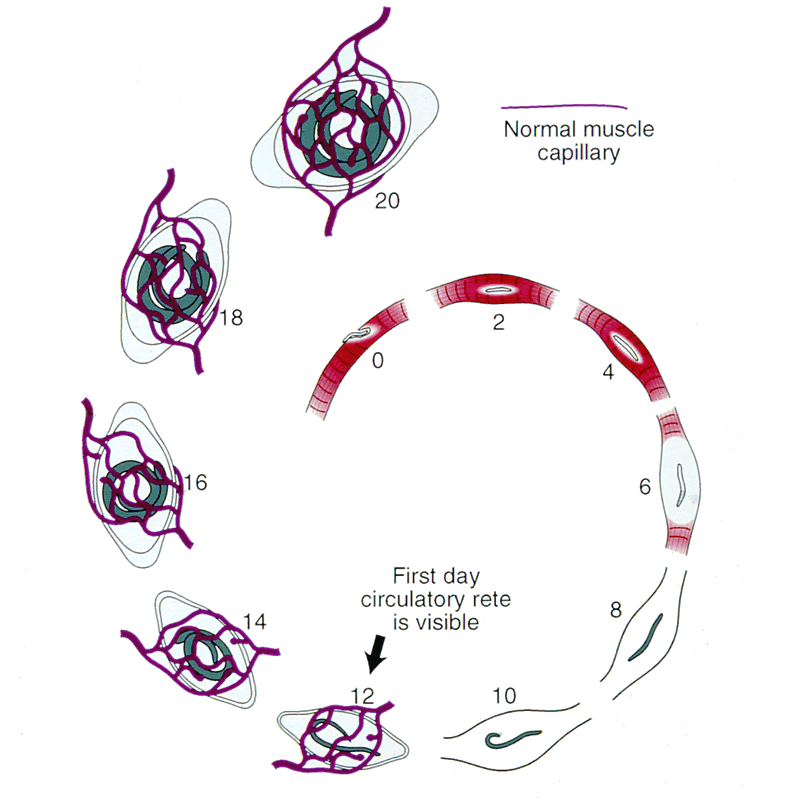
Angiogenesis
Schematic of nurse cell-parasite complex with intact circulatory rete.
Schematic of circulatory rete surrounding a mature nurse cell. India ink preparation of Pagenstecher, 1865.
Circulatory rete surrounding a mature nurse cell of an infected mouse. Note larvae in nurse cells. India ink preparation.
Anatomic plastic cast of mature nurse cells from an infected mouse 30 da post-injection. Circulatory rete is in upper area and normal muscle circulatory system in lower area.
Anatomic plastic cast of mature nurse cells from an infected mouse 30 da post-injection. Note enlarged vessel leading to circulatory rete. Smaller vessels are from normal muscle circulation.
Collagen Capsule Formation
An acellular capsule forms on the outside of the Nurse cell, beginning on Day 10 post-im infection. It continues to thicken until Day 26, and is largely composed of collagen type IV and VI (Polvere, et al, 1998). Collagen synthesis occurs within the cytoplasm of the developing Nurse cell and is directly correlated temporally with the thickening of collagen along the outer surface of the Nurse cell.
Through the use of anti-collagen antibodies and specific RNA probes, the types of collagen present in the capsule were determined. Two dominant collagen types were detected, type IV and type VI. Northern analysis and in situ hybridization studies showed that type IV collagen synthesis begins on Day 10 and ceases on Day 26, while collagen type VI begins on Day 10 and continues throughout the infection period.
Type IV collagen is a large molecule (Wikipedia) and is found throughout the vertebrate body, and most likely functions as a scaffolding element, holding cells in various tissues together. In contrast, collagen type VI is a relatively short molecule (Wikipedia), functioning to bind larger collagen molecules together by cross-linking them, like the individual rungs of a ladder that together support the two legs.
The Nurse cell cytoplasm is infiltrated with numerous, as yet unidentified, mononuclear cells. The number of different types of host cells that gain entrance to the Nurse cell has yet to be determined. Their function(s) in Nurse cell formation and/or maintenance remains enigmatic. Initial observations indicate that the process of cell recruitment into the Nurse cell is ongoing.
Mature Nurse cell-parasite complex. The capsule can be seen above the altered muscle cell cytoplasm.
Mature Nurse cell-parasite complex stained with PAS to detect collagen. Note that worm cuticle also contains collagen. Paraffin section.
Mature Nurse cell-parasite complex stained with antibodies to type IV collagen. Note only the capsule of the nurse cell stains positive. Paraffin section.
Mature Nurse cell-parasite complex stained with antibodies to type VI collagen. Note only the capsule of the nurse cell stains positive. Paraffin section.
Northern analysis of types 4 and 6 collagen RNAs in synchronized infected mouse muscle.
In situ hybridization of sections from mouse tissue 7 days after infection, using a probe for type IV collagen.
In situ hybridization with type IV RNA probe. Day 9 post-im infection.
In situ hybridization of sections from mouse tissue 15 days after infection, using a probe for type IV collagen.
In situ hybridization of sections from mouse tissue 8 months after infection, using a probe for type IV collagen. Note lack of signal.
In situ hybridization of sections from mouse tissue 24 days after infection, using a probe for type VI collagen.
In situ hybridization of sections from mouse tissue 9 days after infection, using a probe for type VI collagen.
In situ hybridization of sections from mouse tissue 15 days after infection, using a probe for type VI collagen.
In situ hybridization of sections from mouse tissue 24 days after infection, using a probe for type VI collagen.
Localization of secreted antigens from L1 during Nurse cell formation
Day 7 post-im infection. Stained with anti-tyvelose antibody. Note staining within developing stichosome and cuticular surface of L1.
Day 9 post-im infection. Stained with anti-tyvelose antibody. Note a light staining of the entire cytoplasm of developing nurse cell, and intense staining of nucleopplasm of each enlarged host cell nucleus.
Day 11 post-im infection. Stained with anti-tyvelose antibody. Note same staining pattern as day 9.
Day 18 post-im infection. Stained with anti-tyvelose antibody. Note intense staining of cytoplasm and nucleoplasm of the now fully developed nurse cell.
180 days post-im infection. Stained with anti-tyvelose antibody. Note pattern of staining is the same as on day 18.